Resource Hub
eProtocol is the foundational building block to enable Clinical Trial Digitization, AI and Automation. Stay on top of the latest trends & best practices to help you with your journey.
What are Digital and eProtocols?
Clinical trials are pivotal for advancing clinical research, yet they still remain complex, resource-intensive, and prone to inefficiencies. At the heart of every clinical trial lies the protocol—the foundational blueprint that dictates the study's objectives, methodology, and operational details. Take a look at the resources here to fast start your journey into Clinical Trial digitization.
- 101: Dive into a primer on the what, why and how eProtocols are digitizing Clinical Trials.
- DIA Global: Read an overview of how Digitizing Protocol Design and Deploying AI to Save Hundreds (of Hours) and Millions (of Dollars) as shared on DIA Global.
- Podcast: Listen to a conversation with Maya Z and Kimberly Tableman on how digital protocols can speed up Clinical Trials.
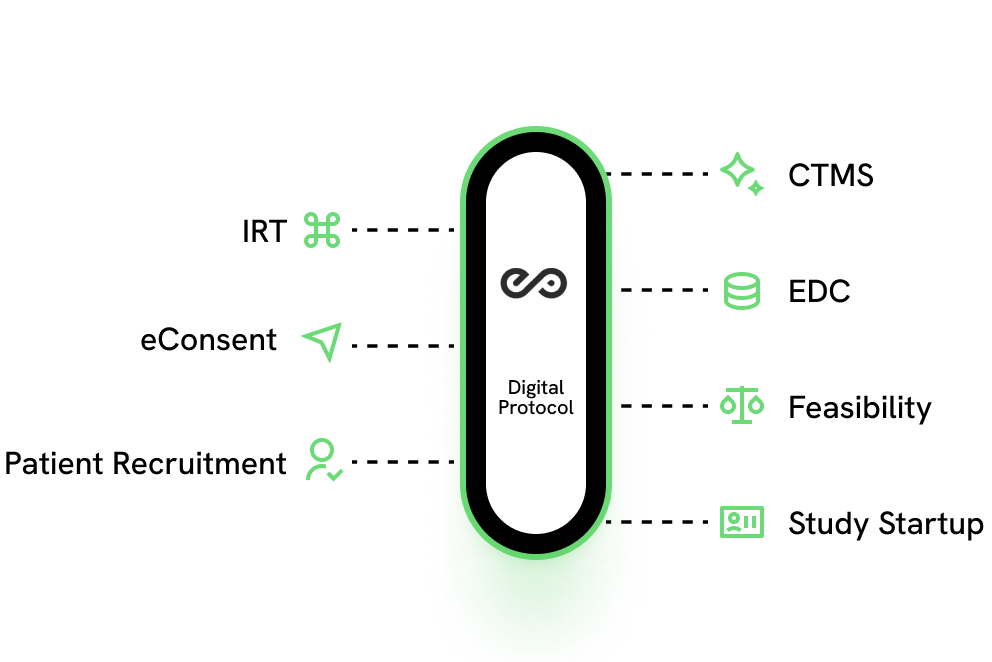
What is Digital Data Flow (DDF)?
Digital Data Flow (DDF) allows clinical trial data to move smoothly between systems, eliminating redundancies and enabling automation through interoperability. A compelling example of this is sending a digitized SOA from a Digital Protocol to feed the EDC for automated eCRF creation.
Women in Clinical Research Scholarship
ESPERO is passionate about empowering women in Clinical Research, amplifying their voices and creating an even playing field in Life Sciences.
Learn more about our 2024 scholarship winner at the link below.

Are you ready to modernize your protocol?
Contact us to learn more about how Espero's digital tools, automation & data insights can improve your protocol.