Digitizing your protocol has never been
Bring your protocol process into the digital age, with digital tools, automation & AI.
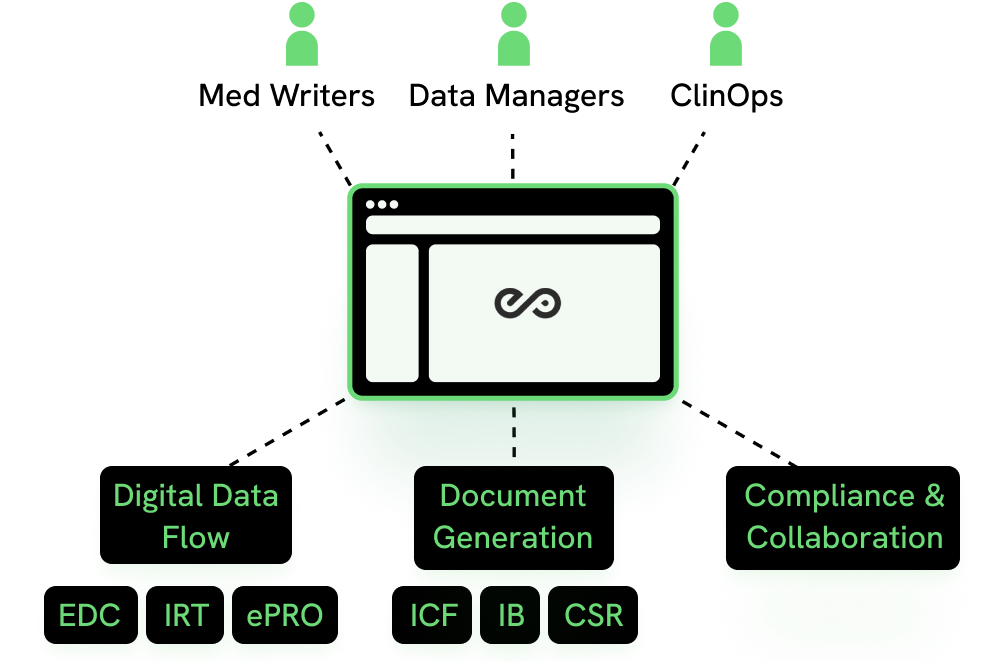
eProtocol, your hub for Clinical Trial efficiency
ESPERO helps you boost your team's efficiency by pre-populating the protocol template, accelerating protocol authoring through a collaborative workflow, and enabling Digital Data Flow by sending the digital protocol to downstream systems.
- Centralized processes and workflows allows for tracking, audit trail, collaboration and protocol system of record
- Data-driven protocol design with real-time insights with patient and site feedback for reduced burden
- Automated FDA Submission and aligned with ICH-M11
- Dynamically generated study documents with 1-click ICF, IB, CSR, SAP, and Lab Manual document generation
- SOA is fed direct to EDC, reducing EDC build timelines by 67 days.
Collaborative Protocol Authoring
Streamline your protocol process for maximum productivity and compliance.
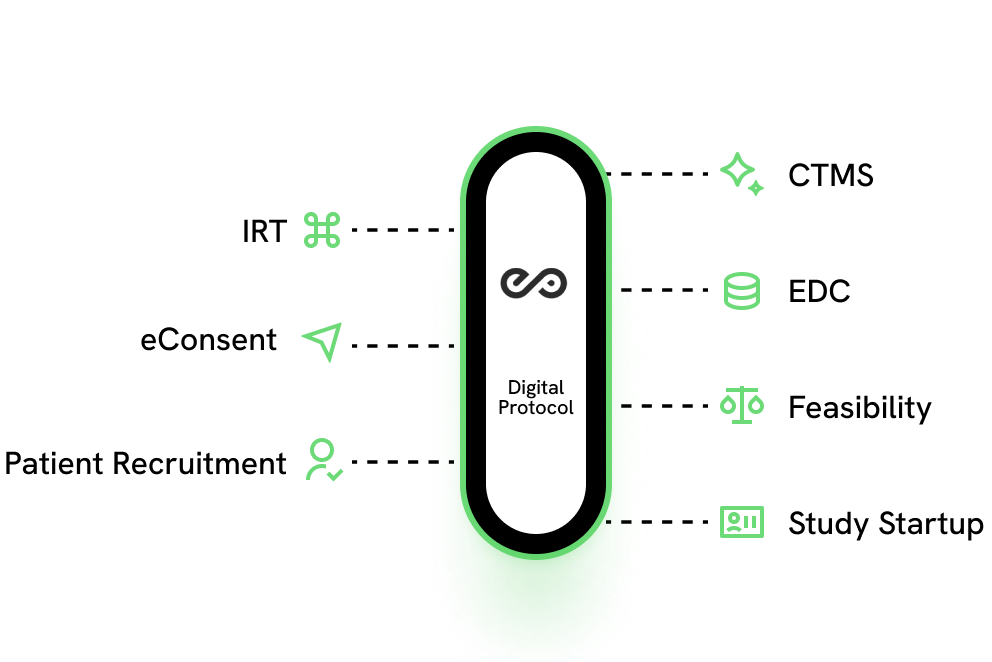
Designed to enable Digital Data Flow (DDF)
Downstream system integration to the EDC, IRT and other study systems shouldn't be an afterthought. ESPEROs Insight Driven Protocol Platform helps accelerate study startup timelines and overcome the challenges of implementing Protocol Amendments.
Efficiency and Quality, no comprises.
Insight data-driven protocols with a click of the button, help you deliver a higher quality protocol, faster.
With just a few keywords, ESPERO's machine learning and advanced analytics will analyze 100's of historical clinical trials, and curate insights and recommendations for your study. Leveraging the latest in Generative-AI, ESPERO helps your team reduce protocol design time by 30%, and get your team all on the same page, literally.
Why Espero?

Automated FDA submission
Collaborative workflow
Reduced Patient and Site burden

eCRF automation (DDF)

Dynamically generate study documents

Cost forecasting
Frequently asked questions
What is a Digital Protocol and/or eProtocol?
A Digital Protocol, or eProtocol, is a fully digital representation of a clinical trial protocol. It enables seamless data exchange across systems, reduces manual effort, and accelerates study startup by standardizing and automating key processes. A more comprehensive overview of what Digital Protocols are on can be found here.
What is Digital Data Flow (DDF), and why does it matter in clinical trials?
Digital Data Flow (DDF) allows clinical trial data to move smoothly between systems, eliminating redundancies and manual re-entry. A compelling example of this is sending a digitized SOA to feed the EDC for automated eCRF creation. More information on DDF can be found at the industry collaboration CDISC website. Or read this primer on "The role of ICH-M11 and Digital Data Flow (DDF)".
What is the ICH M11 guideline, and how does it impact protocol development?
The ICH M11 guideline establishes a global standard for clinical trial protocols, ensuring consistency and improving collaboration between sponsors, regulators, and researchers. By adopting ICH M11, the industry can streamline regulatory submissions and enable greater efficiency in protocol design and execution.
How does ESPERO’s eProtocol platform improve clinical trial processes?
ESPERO's eProtocol platform modernizes protocol development by digitizing and automating workflows, reducing timelines, and improving data quality. It supports compliance with ICH M11 and integrates seamlessly with downstream systems to simplify trial execution.
Are you ready to modernize your protocol?
Contact us to learn more about how Espero's digital tools, automation & data insights can improve your protocol.